
Interdisciplinary Science Blog


Guided by Art: An In-Depth Exploration of Compounds, Colors, Sound, and Calculations
By Jennifer Staple-Clark, Founder and Executive Director
Art, history, chemistry, music, physics, mathematics, and geology converged in a captivating interdisciplinary exploration that reached its pinnacle at the Yale Art Gallery. Witnessing the enthusiasm, curiosity, and excitement of our students throughout the journey was exhilarating.
Our interdisciplinary journey began with a deep dive into geology, soil, cave art, Maya art, and chemistry. Students observed various rocks and their associated compounds, drawing connections between the reddish hues of sandstone rock and Holyoke soil collected from our campus, both containing Iron Oxide (Fe2O3) responsible for the red coloration. Last year, the students collected those soil samples and marked them on the campus map, and this time they contemplated the composition and colors of the soils. They also considered the colors of other rocks, including Quartz's Silicon Dioxide (SiO2) and Pink Granite’s Potassium Feldspar (KAlSi3O8). This exploration led us to ponder the pigments made of natural materials that were used thousands of years ago. Our Environmentalist and resident geologist, Grace Kenney, as well as our Horticulturalist, Rana Bates, joined on these explorations.
There are many incredible books that became resources for contemplating the science and history of color, including Victoria Finlay’s The Brilliant History of Color in Art. The students heard the story of the discovery of the Lascaux cave art by a group of children in 1940, and how the pigments have faded from exposure to air and humidity. “The paintings that had been preserved in darkness for so many thousands of years became almost invisible within just 20. And this is another element of the history of colors in art: they are there, and then they go. They do not stay the same, and when you look at a painting, you’re also, in a tiny way, changing it” (Finlay 11). We discussed red ochre, which is also Fe2O3, and other pigments used in cave art. We also thought about the Maya Blue pigment derived from the Indigo plant, and we looked at the chemical structure of the Indigo compound, with its many carbon atoms. Along the way, students calculated the atomic weights of the compounds of these natural materials that we were discussing.
Transitioning from ancient art to the Renaissance era, we explored the paint pigments used by renowned artists like Vermeer, Holbein, Durer, and Steen. Their paintings featuring musical instruments prompted a seamless fusion of music and art history. The students noticed some familiar instruments in the paintings, as well as some unique instruments that they haven't seen before, such as the Oud in Holbein's The Ambassadors. Here, music and art history meshed as the students considered sound, and how and why we hear musical instruments.
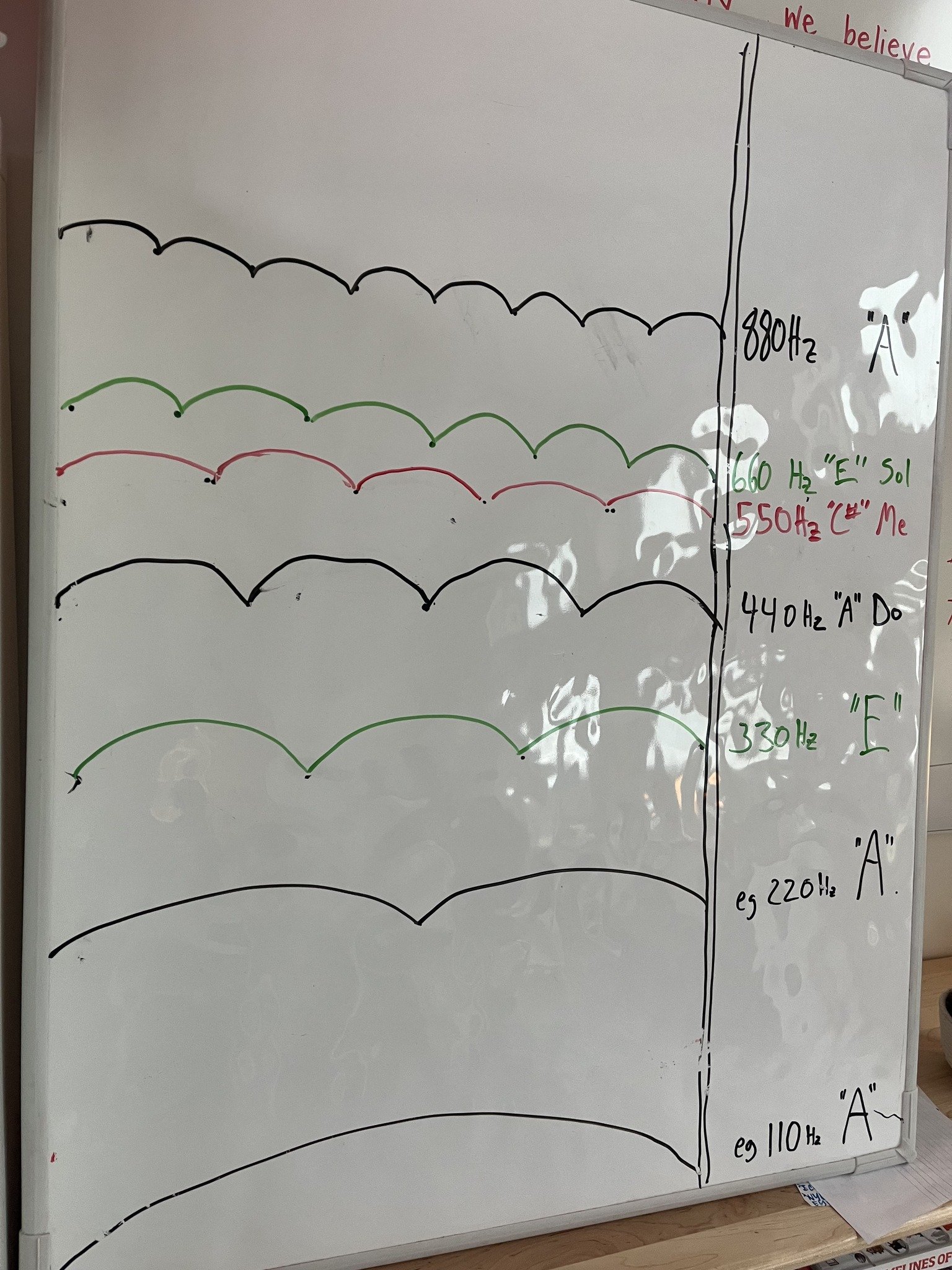
Before we dove into sound waves, we first thought together about what makes solids, liquids, and gasses, and the students role played the gaseous air molecules all around us to understand the movement of air molecules. They connected this to the vibrations that happen when instruments are being played and produce sound waves. Our music educator Eben Pariser joined me to demonstrate how different strings on the guitar as well as keys on the piano produce sound waves of different frequencies, which we can measure with the units Hertz (Hz), or cycles per second. As notes on the piano were played, our students measured the frequency of each note. After collecting six data points for the frequency of A2 on the piano using a Hz meter, our students found the average frequency. Then they took six more readings for the frequency of A3 on the piano, an octave higher. They compared the frequency from their average readings to the mathematically predicted frequencies for A 440Hz tuning, and also began considering the frequency of sound waves coming from a recorder, too.
On a subsequent day, our students arrived to an enticement where the table was set up with a sandstone rock, a picture of red blood cells, a picture of Mars, and the painting The Little Street by Vermeer. They wrote observations and wonderings about each item, and thought about what element all of these might have in common. From their prior exploration of red ochre, the students deduced the common element of red-causing iron in these various forms and uses. Later that day, we returned to Vermeer, Holbein, Steen, and Durer. A student scribed a multitude of paint names and compound formulas used by these artists. Students figured out the elements and symbols from the periodic table in each of the pigment compounds. We learned about Lead White, Vermilion Red, Ultramarine Blue, Umber, and so many more paints used by artists hundreds of years ago. Then our students calculated the atomic weights for each of the pigment compounds, such as determining that Umber has an atomic weight of 554 amu.
Continuing our interdisciplinary approach, the students explored the works of abstract artist Pier Mondrian. Each student crafted their own Mondrian-inspired artwork, incorporating mathematical calculations of shape areas.
The exploration and appreciation for a variety of forms of art brought us down a multitude of extraordinary paths, with student wonderings and curiosity leading the way. Student questions led us to think about the origin of element names, consider the field of archaeology and discoveries of artifacts from long ago, and so much more. Culminating our interdisciplinary journey at the Yale Art Gallery, students applied their integrated knowledge, recognizing that compounds are the essence behind the colors in masterful artworks throughout history.
Citations:
Finlay, Victoria. The Brilliant History of Color in Art. Getty Publications, 2014.
About The Blog Author, Jennifer Staple-Clark
For nearly 25 years, Jennifer Staple-Clark has been a leader in nonprofit innovation, and she currently leads two nonprofit organizations: Unite For Sight and Slate School. In 2000, Jennifer, who was then a sophomore at Yale University, founded Unite For Sight in her dorm room. Unite For Sight is now a leader both in global health education and in providing cost-effective care to the world's poorest people.
A cum laude graduate of Yale University, Jennifer received a Bachelor of Science Degree in Anthropology as well as in Molecular, Cellular, and Developmental Biology. Early in her career, Jennifer taught high school environmental science and chemistry, where she brought project-based learning to the independent school where she was teaching. She received a national educator award for her work. After dedicating herself to being a high school educator, Jennifer completed two years of medical school at Stanford University School of Medicine before shifting to pursue her passionate interest in global health and eliminating patient barriers to care through building the nonprofit Unite For Sight into an organizational leader in the field. After more than a decade dedicated solely to Unite For Sight, in 2017, Jennifer founded Slate School, an innovative 501(c)3 nonprofit K-12 curiosity-driven and nature-based school in Connecticut. As part of her work as Founder and Executive Director of Slate School, Jennifer draws on her science, medical, anthropology, and public health background to integrate curiosity-driven, interdisciplinary chemistry, biology, and physics into the Upper Elementary classrooms. She is also closely involved in the Upper School interdisciplinary curriculum development.